Abstract
Introduction: Therapy options for chronic lymphocytic leukemia (CLL) are rapidly changing with the approval of ibrutinib, an irreversible Bruton's tyrosine kinase inhibitor, and venetoclax, a Bcl2 inhibitor. In the RESONATE studies, ibrutinib showed significant improvements in overall response rates (ORR), progression-free-survival (PFS), and overall survival (OS). Nonetheless, complete response (CR) rates were only about 4%.
During the first few weeks of treatment, ibrutinib will mobilize CLL cells from tissue sites into peripheral blood. Rituximab, a chimeric monoclonal CD20 antibody, when used with ibrutinib, has shown to immediately clear CLL from the blood with durable responses but with CR's of only 8%.
Venetoclax is approved for relapsed or refractory CLL with 17p deletion. In a phase 2 study, at a median follow-up of 12.1 months, the ORR was ~79%, but with only 16% CR's. Amongst the 45 patients of whom minimal residual disease (MRD) was assessed, 24 were negative for MRD.
In vitro studies have shown that nurse-like cells (NLCs) will protect CLL cells against spontaneous apoptosis by producing chemokines and interleukins such as SDF1, suggestive of a mechanism of ibrutinib resistance. SDF1 was shown to bind to its receptor CXCR4 located on CLL cells and activate a signal transduction pathway that would reduce spontaneous apoptosis. Dexamethasone was found to decrease NLC viability. This suggests that a combined therapy of ibrutinib plus steroids along with rituximab and venetoclax may be necessary to achieve deeper CR's in high-risk CLL.
In addition, CLL with overexpression of exosome miR-155 is associated with a poor response to chemoimmunotherapy. In prior preclinical studies, exosome miR-150 and miR-155 have been shown to increase with BCR activation via α-IgM stimulation. We hope that via next generation microRNA sequencing from our patients' CLL plasma cells we will observe a decrease in the levels of exosome miR-150 and miR-155 in response to inhibition of BCR-pathway by ibrutinib as seen in preclinical studies.
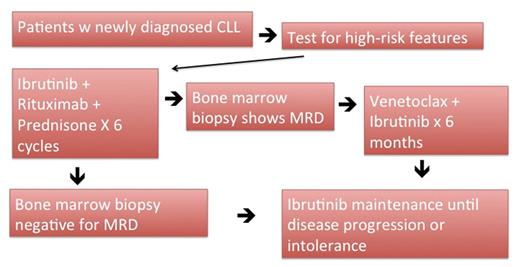
Methods: Patients with newly diagnosed high-risk CLL per FISH studies will be enrolled. Ibrutinib will be administered orally 420 mg daily, rituximab will be dosed at 375 mg/m2 intravenously on days 1, 8, 15, 22 of cycle 1 and on day 1 for cycle 2-6. Prednisone will be administered as 10 mg one week on, one week off per cycle. Each cycle will last 28 days. After 6 cycles, if patients have MRD, venetoclax will be added in on a weekly schedule with weekly ramp up from 20mg to 50mg, 100mg, and 200mg for a total duration of 6 months plus ibrutinib at 420mg as maintenance until disease progression or drug intolerance. There is the option to reintroduce venetoclax, prednisone, or rituximab if patients progress during maintenance.
Results: The primary objectives of this study are to evaluate safety and MRD negative CR rates based on IWG-CLL criteria. For the evaluation of safety, the proportions of the 29 subjects with any adverse events, each specific adverse event, and study-related adverse events will be reported along with the 95% confidence intervals. The proportion of the 29 subjects who demonstrate a MRD negative CR will be reported along with the 95% confidence interval. A sample size of 29 will produce a two-sided 95% confidence interval with a width≤ 0.380.
In addition, Kaplan-Meier method will be performed to evaluate time to first MRD negativity (TTMN), time to first relapse (TTFR), PFS and OS, respectively. Based on the Kaplan-Meier curve, the median time to TTMN, TTFR, PFS and OS, respectively, will be reported along with the 95% confidence interval if it exists. Correlation coefficients will be derived to describe the relationship between continuous endpoints and Kendall's tau will be derived to describe the relationship between categorical endpoints.
There is no planned interim analysis for the CR rate. However, at any time point if an excessive number of adverse events are observed, the study will be terminated.
Conclusion: In this Phase IB/II clinical trial, we seek to observe prolonged response rates of ibrutinib via inhibiting NLC's by adding prednisone. Also, we anticipate that rituximab will further amplify the CR's. For maintenance therapy, we hope that by combining venetoclax with ibrutinib in high-risk CLL patients who were unable to achieve negative MRD's, we will be able to induce deeper remissions.
Persky: Genentech: Consultancy; MorphoSys: Other: Independent Data Monitoring Committee member ; Verastem: Consultancy; Spectrum Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal